Home > E. Pathology by systems > Respiratory system > Lungs > pulmonary nodular lymphoid hyperplasia
pulmonary nodular lymphoid hyperplasia
Monday 14 March 2005
PNLH, pulmonary pseudolymphoma; PNLH, pseudolymphoma of the lung
Digital slides
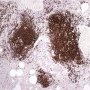
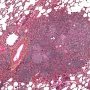
![]() HPC:72: pulmonary nodular lymphoid hyperplasia.
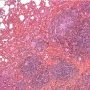
HPC:72: pulmonary nodular lymphoid hyperplasia.![]() JRC:19395 : Pulmonary pseudolymphoma.
JRC:19395 : Pulmonary pseudolymphoma.
Definition: Pulmonary nodular lymphoid hyperplasia (PNLH) is defined as reactive lymphoid proliferation forming solitary or multiple nodules or localized infiltrates localized in the lungs.
Pulmonary nodular lymphoid hyperplasia (NLH) is a term first suggested by Kradin and Mark to describe one or more pulmonary nodules or localized lung infiltrates consisting of reactive lymphoid proliferation.
Pulmonary nodular lymphoid hyperplasia (PNLH) is a rare lung disease classified as a non-neoplastic pulmonary lymphoid lesion. It is believed that 36 % of cases present with multifocal lesions.
Radiological presentations are generally solitary or multiple nodules, but air bronchograms and ground glass attenuation may be present.
Patients mostly asymptomatic and the lesions were detected coincidentally on routine chest X-rays.
Nodular lymphoid hyperplasia consists of a reactive nodular lymphoid proliferation that presents as 1 or more pulmonary masses.
Most cases diagnosed as pulmonary “pseudolymphomas” in the past would now be considered low-grade B-cell lymphoma of BALT.
Nonetheless, nodular lymphoid hyperplasia refers to those cases that are reactive when stringent histologic, immunophenotypic, and genotypic criteria are applied.
Clinical Features
Most patients are asymptomatic and present with a nodule or nodules identified incidentally on chest x-ray. When symptoms are present, they are nonspecific and include cough, dyspnea, and pleuritic chest pain.
Nodular lymphoid hyperplasia occurs slightly more often in females than males by a ratio of 4:3. Patients range in age from 19 to 80 years (median, 60 years). Mediastinal or hilar adenopathy may be present in approximately one-third of patients.
Although initial reports did not suggest an association with collagen vascular disease, a more recent report61 has identified nodular lymphoid hyperplasia in a patient with Sjögren syndrome.
Macroscopy
Gross features of nodular lymphoid hyperplasia consist of well-circumscribed gray to white tan nodule(s). Multiple nodules are present in one-third of cases. The nodules are often subpleural but may also be peribronchial.
Microscopy
Histologically, nodular lymphoid hyperplasia is well demarcated and composed of numerous reactive germinal centers with sheets of interfollicular plasma cells.
Reactive germinal centers may be present within alveolar septa. Bégueret and colleagues identified lymphoepithelial lesions in 60% of their cases, although these lesions were not present in the series of Abbondanzo et al.
Interfollicular fibrosis is often present and may be extensive, partially effacing the underlying pulmonary parenchyma. Rare cases may contain scattered giant cells.
Dutcher bodies, bronchial cartilage invasion, or plaquelike involvement of the pleura are not identified.
Immunochemistry
Immunohistochemical and molecular studies may be helpful in distinction from low-grade B-cell lymphoma.
The reactive lymphoid follicles stain positively for B-cell markers (CD20 and CD79a), while interfollicular lymphocytes stain predominantly for T-cell markers (CD3, CD43, and CD5).
Unlike many cases of low-grade B-cell lymphoma, coexpression of CD20 and CD43 by lymphocytes is not identified either within the parenchyma or in lymphoepithelial lesions.
Expression of BCL-2 is absent in germinal centers and limited to mantle zone and interfollicular lymphocytes. A polyclonal pattern of expression is present in interfollicular plasma cells on stains for κ and λ light chains.
A polyclonal pattern is also present on PCR analysis for the immunoglobulin heavy chain gene.
Synopsis
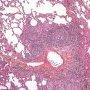
![]() expansive and circumscribed nodular lymphoid aggregations along the course of bronchovascular bundles
expansive and circumscribed nodular lymphoid aggregations along the course of bronchovascular bundles![]() numerous confluent reactive lymphoid follicles
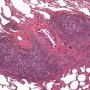
numerous confluent reactive lymphoid follicles![]() well demarcated mass (or masses) consisting of numerous reactive lymphoid follicles with germinal centers
well demarcated mass (or masses) consisting of numerous reactive lymphoid follicles with germinal centers![]() sheets of interfollicular plasma cells
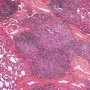
sheets of interfollicular plasma cells![]() plasma cells are polyclonal on kappa / lmabda light chain staining
plasma cells are polyclonal on kappa / lmabda light chain staining![]() interfollicular fibrosis +/-
interfollicular fibrosis +/-![]() sharp demarcation from normal parenchyma
sharp demarcation from normal parenchyma![]() follicular lymphoid hyeprplasia
follicular lymphoid hyeprplasia![]() interfollicular lymphoplasmocytosis
interfollicular lymphoplasmocytosis![]() extension along lobular septa and in interalveolar septa
extension along lobular septa and in interalveolar septa![]() no destructive lymphoepithelial lesions
no destructive lymphoepithelial lesions![]() no Dutcher bodies (eosinophilic intranuclear deposits of immunoglobulin)
no Dutcher bodies (eosinophilic intranuclear deposits of immunoglobulin)![]() no follicular colonization by atypical lymphocytes
no follicular colonization by atypical lymphocytes
Associations
![]() dysimmunity - autoimmune disorders
dysimmunity - autoimmune disorders
![]() AIDS
AIDS
Differential diagnosis
![]() simple BALT hyperplasia (follicular bronchiolitis)
simple BALT hyperplasia (follicular bronchiolitis)![]() lymphoid interstitial pneumonia (LIP)
lymphoid interstitial pneumonia (LIP)
The differential diagnosis of nodular lymphoid hyperplasia includes LIP, follicular bronchiolitis, and low-grade B-cell lymphoma. These disorders can be distinguished by consideration of the clinical, radiographic, and histologic features.
Radiographically, nodular lymphoid hyperplasia consists of well-demarcated nodules or masses in contrast to the diffuse reticulonodular or small nodular infiltrate of LIP. Histologically, there is a confluent proliferation of lymphoid follicles in nodular lymphoid hyperplasia. The infiltrate in LIP, on the other hand, diffusely expands the interstitium. Reactive lymphoid follicles are a more prominent and consistent feature in nodular lymphoid hyperplasia.
Follicular bronchiolitis is also distinct. Radiographically, bilateral centrilobular and sometimes peribronchial nodules are present on CT scan. In contrast, the nodules present in nodular lymphoid hyperplasia are generally larger, well demarcated, and often solitary with a subpleural predilection. Histologically, follicular bronchiolitis shows lymphoid follicles with reactive germinal centers in a peribronchial or peribronchiolar distribution. As with features on imaging studies, a mass or nodule is not present histologically.
Distinction of nodular lymphoid hyperplasia from low-grade B-cell lymphoma may be more problematic. Imaging studies of both nodular lymphoid hyperplasia and low-grade B-cell lymphoma are similar and show 1 or more well-defined nodules.
Histological, immunohistochemical, and molecular features, however, usually allow their separation. In nodular lymphoid hyperplasia, in contrast to BALT lymphoma, cases show only focal (not extensive) tracking of lymphatics and usually no plaquelike involvement of the pleura.
Coexpression of CD20 and CD43 by lymphocytes may sometimes be present in low-grade B-cell lymphoma but is not identified in nodular lymphoid hyperplasia. Plasma cells in nodular lymphoid hyperplasia show a polyclonal pattern of immunohistochemical staining for κ and λ chains.
On the other hand, light chain restriction is identified in a significant proportion of cases of low-grade B-cell lymphoma. Likewise, immunoglobulin heavy chain gene rearrangement can often be demonstrated in low-grade BALT lymphoma, but it is not present in nodular lymphoid hyperplasia.
Pathogenesis
The pathogenesis of nodular lymphoid hyperplasia is unknown. The observation of a subpleural predilection, similar to intrapulmonary lymph nodes, suggests the possibility of an aberrant organization of BALT, possibly reflecting the effects of an antecedent inflammatory event.
Unlike follicular bronchiolitis and LIP, most cases appear unassociated with connective tissue disease, immunodeficiency, or prior viral infection.
Prognosis and Treatment
Nodular lymphoid hyperplasia is a benign lesion. Surgical excision is generally curative.
References
![]() A case of pulmonary nodular lymphoid hyperplasia with a resected cavity, followed by spontaneous regression of the remaining lesions. Miyoshi S, Hamada H, Katayama H, Hamaguchi N, Irifune K, Ito R, Ohtsuki Y, Yoshino T, Higaki J. Intern Med. 2010;49(15):1617-21. PMID: 20686301 (Free)
A case of pulmonary nodular lymphoid hyperplasia with a resected cavity, followed by spontaneous regression of the remaining lesions. Miyoshi S, Hamada H, Katayama H, Hamaguchi N, Irifune K, Ito R, Ohtsuki Y, Yoshino T, Higaki J. Intern Med. 2010;49(15):1617-21. PMID: 20686301 (Free)
![]() Nodular lymphoid hyperplasia of the lung: the role of positron emission tomography in diagnosis. Yilmaz U, Unsal I, Halilçolar H, Anar C, Yildirim Y, Sanli A, Kargi A. Tuberk Toraks. 2009;57(4):417-21. PMID: 20037858 [Free]
Nodular lymphoid hyperplasia of the lung: the role of positron emission tomography in diagnosis. Yilmaz U, Unsal I, Halilçolar H, Anar C, Yildirim Y, Sanli A, Kargi A. Tuberk Toraks. 2009;57(4):417-21. PMID: 20037858 [Free]
![]() Nodular lymphoid hyperplasia: rare case of lymphoproliferative disease in the lung. Karube Y, Chida M, Honma K, Araki O, Kobayashi S, Miyoshi S. Gen Thorac Cardiovasc Surg. 2009 Jun;57(6):324-7. PMID: 19533282
Nodular lymphoid hyperplasia: rare case of lymphoproliferative disease in the lung. Karube Y, Chida M, Honma K, Araki O, Kobayashi S, Miyoshi S. Gen Thorac Cardiovasc Surg. 2009 Jun;57(6):324-7. PMID: 19533282
![]() Pulmonary nodular lymphoid hyperplasia associated with Sjögren’s syndrome. Song MK, Seol YM, Park YE, Kim YS, Lee MK, Lee CH, Jeong YJ. Korean J Intern Med. 2007 Sep;22(3):192-6. PMID: 17939337
Pulmonary nodular lymphoid hyperplasia associated with Sjögren’s syndrome. Song MK, Seol YM, Park YE, Kim YS, Lee MK, Lee CH, Jeong YJ. Korean J Intern Med. 2007 Sep;22(3):192-6. PMID: 17939337
![]() Nodular lymphoid hyperplasia of the lung: a very rare disease entity. Sakurai H, Hada M, Oyama T. Ann Thorac Surg. 2007 Jun;83(6):2197-9. PMID: 17532425
Nodular lymphoid hyperplasia of the lung: a very rare disease entity. Sakurai H, Hada M, Oyama T. Ann Thorac Surg. 2007 Jun;83(6):2197-9. PMID: 17532425
![]() Multifocal nodular lymphoid hyperplasia of the lung. Kajiwara S, Sakai S, Soeda H, Takahashi N, Okafuji T, Yoshimitsu K, Yabuuchi H, Yoshino I, Honda H. J Thorac Imaging. 2005 Aug;20(3):239-41. PMID: 16077344
Multifocal nodular lymphoid hyperplasia of the lung. Kajiwara S, Sakai S, Soeda H, Takahashi N, Okafuji T, Yoshimitsu K, Yabuuchi H, Yoshino I, Honda H. J Thorac Imaging. 2005 Aug;20(3):239-41. PMID: 16077344
![]() Nodular lymphoid hyperplasia in the lung. Kawahara K, Shiraishi T, Okabayashi K, Iwasaki A, Hayashi K, Matsuo T, Mita S, Maekawa T, Shirakusa T, Kikuti M, Tashiro K. Thorac Cardiovasc Surg. 1996 Aug;44(4):210-2. PMID: 8896166
Nodular lymphoid hyperplasia in the lung. Kawahara K, Shiraishi T, Okabayashi K, Iwasaki A, Hayashi K, Matsuo T, Mita S, Maekawa T, Shirakusa T, Kikuti M, Tashiro K. Thorac Cardiovasc Surg. 1996 Aug;44(4):210-2. PMID: 8896166
![]() Familial pulmonary nodular lymphoid hyperplasia. Franchi LM, Chin TW, Nussbaum E, Riker J, Robert M, Talbert WM. J Pediatr. 1992 Jul;121(1):89-92. PMID: 1625100
Familial pulmonary nodular lymphoid hyperplasia. Franchi LM, Chin TW, Nussbaum E, Riker J, Robert M, Talbert WM. J Pediatr. 1992 Jul;121(1):89-92. PMID: 1625100
![]() Abbondanzo, S. L. , W. Rush , K. E. Bijwaard , and M. N. Koss . Nodular lymphoid hyperplasia of the lung: a clinicopathologic study of 14 cases. Am J Surg Pathol 2000. 24 (4):587–597.
Abbondanzo, S. L. , W. Rush , K. E. Bijwaard , and M. N. Koss . Nodular lymphoid hyperplasia of the lung: a clinicopathologic study of 14 cases. Am J Surg Pathol 2000. 24 (4):587–597.
![]() Bégueret, H. , B. Vergier , M. Parrens , et al. Primary lung small B-cell lymphoma versus lymphoid hyperplasia: evaluation of diagnostic criteria in 26 cases. Am J Surg Pathol 2002. 26 (1):76–81.
Bégueret, H. , B. Vergier , M. Parrens , et al. Primary lung small B-cell lymphoma versus lymphoid hyperplasia: evaluation of diagnostic criteria in 26 cases. Am J Surg Pathol 2002. 26 (1):76–81.